Similarities and Differences in the Electron Density of Cumulated and Conjugated Systems
- Julio Hernández
- Flash-Talks 2 , Chemistry
- Wednesday, July 16, 2025
- 12:14 pm
Julio César Hernández Camacho, D.Sc. José Enrique Barquera Lozada
Instituto de Química, Universidad Nacional Autónoma de México, Circuito exterior, Ciudad Universitaria, Coyoacán, 04510 Ciudad de México, CDMX.
e-mail: crazieeyesh@gmail.com
[n]cumulenes are unsaturated linear hydrocarbons which possess “n” continuous C=C double bonds and “n+1” carbon atoms. This unique characteristic makes them promising candidates for applications, such as: molecular wires, nonlinear optical materials and organic field-effect transistors (OFETs). However, the particular π electronic structure of [n]cumulenes varies according to their terminal functional groups and also if the number of cumulated double bonds is even or odd.
Therefore, a set of QTAIM calculations were conducted to shed light on the nature of bonds in even and odd cumulated systems, with n = 3, 4, 5 and 6, functionalized with alkyls, aryls and N-heterocyclic carbenes (NHCs). Through these calculations, variations in the delocalization indexes and in the values of the Laplacian of the electron density at the critical point of the bonds present in the cumulenic chain were obtained. Noteworthy, the most outstanding changes were observed on the ellipticity of the even systems functionalized with NHCs. Finally, the results were compared with polyynes and polyenes, because they are also conjugated hydrocarbons, structurally similar but more widely studied.
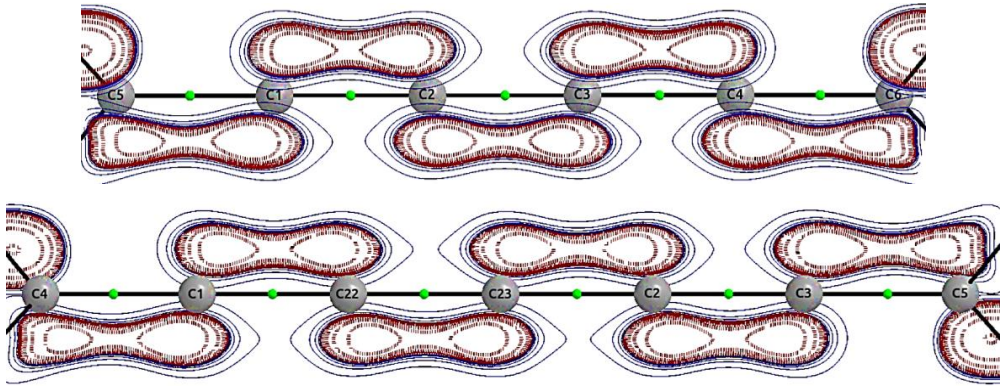
From top to bottom, contour plots of the Laplacian of the electron density are shown for the compounds: tetramethyl-[5]cumulene and tetramethyl-[6]cumulene, respectively.
Figure 1 From top to bottom, contour plots of the Laplacian of the electron density are shown for the compounds: tetramethyl-[5]cumulene and tetramethyl-[6]cumulene, respectively.
References:
[1] Januszewski, J. A., & Tykwinski, R. R. (2014). Synthesis and properties of long [n] cumulenes (n≥ 5). Chemical Society Reviews, 43(9), 3184-3203.
[3] Barquera‐Lozada, J. E. (2020). How to bend a cumulene. Chemistry–A European Journal, 26(20), 4633-4639.